Navigating The Regulatory Landscape: FDA Requirements For Skin Care Products
Navigating the Regulatory Landscape: FDA Requirements for Skin Care Products
Related Articles: Navigating the Regulatory Landscape: FDA Requirements for Skin Care Products
Introduction
With great pleasure, we will explore the intriguing topic related to Navigating the Regulatory Landscape: FDA Requirements for Skin Care Products. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Navigating the Regulatory Landscape: FDA Requirements for Skin Care Products
The skin care industry, a multi-billion dollar market, is teeming with products promising everything from wrinkle reduction to flawless complexion. However, amidst this plethora of options, navigating the regulatory landscape can be challenging. The Food and Drug Administration (FDA) plays a crucial role in ensuring the safety and efficacy of these products, establishing a framework that protects consumers from potentially harmful ingredients and misleading claims.
This article provides a comprehensive overview of FDA requirements for skin care products, outlining key regulations, highlighting areas of focus, and emphasizing the importance of compliance for both manufacturers and consumers.
Defining the Scope: What is a Skin Care Product?
The FDA classifies skin care products as cosmetics, which are defined as articles intended to be rubbed, poured, sprinkled, or sprayed on, introduced into, or otherwise applied to the human body for cleansing, beautifying, promoting attractiveness, or altering the appearance without affecting the body’s structure or function. This broad definition encompasses a wide range of products, including:
- Cleansers: Products designed to remove dirt, oil, and makeup from the skin.
- Toners: Solutions used to balance the skin’s pH and prepare it for moisturizers.
- Moisturizers: Products that hydrate and soften the skin.
- Serums: Concentrated formulas containing active ingredients like vitamins, antioxidants, and growth factors.
- Masks: Treatments applied to the skin for a specific period to address various concerns.
- Sunscreens: Products designed to protect the skin from harmful ultraviolet (UV) radiation.
- Anti-aging products: Products formulated to address signs of aging like wrinkles, fine lines, and age spots.
- Acne treatments: Products intended to reduce acne breakouts and blemishes.
The FDA’s Regulatory Framework: A Balancing Act
The FDA’s approach to regulating cosmetics is based on a "self-regulatory" model. This means that manufacturers are primarily responsible for ensuring the safety and labeling of their products. However, the FDA retains the authority to take action against products that pose a health risk or are mislabeled.
Key Requirements for Skin Care Products:
- Safety: Manufacturers must ensure that their products are safe for intended use when applied as directed. This involves conducting appropriate testing to identify potential risks and ensuring that ingredients are not harmful or adulterated.
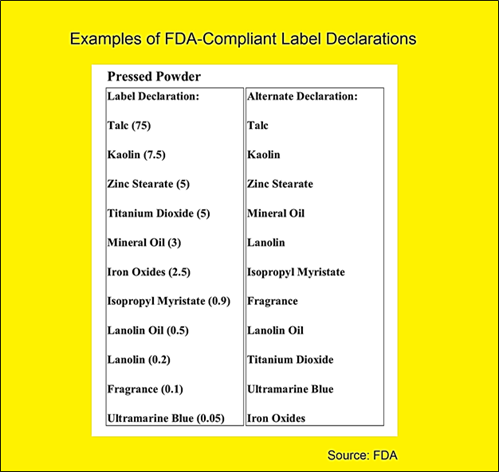
- Labeling: The FDA requires accurate and truthful labeling of cosmetics. This includes listing all ingredients in descending order of predominance, providing a clear statement of the product’s intended use, and including appropriate warnings if necessary.
- Claims: Manufacturers are prohibited from making false or misleading claims about the efficacy of their products. Claims must be supported by scientific evidence and cannot be exaggerated or deceptive.
- Good Manufacturing Practices (GMPs): The FDA encourages manufacturers to follow GMPs to ensure consistent product quality and safety. GMPs encompass a range of practices, including quality control procedures, sanitation, and record-keeping.
Focus Areas: Key Concerns and Regulations
The FDA’s focus on skin care product regulation extends beyond general safety and labeling requirements. Several areas receive particular attention due to their potential for harm or consumer confusion:
- Sunscreens: The FDA has stringent regulations for sunscreens, requiring specific testing to ensure effectiveness in blocking UV radiation. Manufacturers must also comply with labeling requirements regarding SPF values and broad-spectrum protection.
- Anti-aging products: The FDA scrutinizes claims related to anti-aging products, particularly those claiming to reduce wrinkles or reverse signs of aging. Manufacturers must provide scientific evidence to support such claims.
- Cosmetic ingredients: The FDA maintains a list of prohibited and restricted ingredients in cosmetics. This list is updated regularly to address emerging safety concerns.
- Product recalls: The FDA has the authority to issue product recalls if a product is found to be unsafe or mislabeled. Manufacturers are obligated to cooperate with the FDA in the event of a recall.
Understanding the Importance of FDA Regulations
The FDA’s role in regulating skin care products is critical for several reasons:
- Protecting Consumers: By establishing safety standards and enforcing labeling requirements, the FDA helps protect consumers from potentially harmful products.
- Ensuring Efficacy: The FDA’s scrutiny of product claims helps ensure that consumers are not misled about the effectiveness of products.
- Maintaining Public Health: The FDA’s efforts to prevent the spread of infections and diseases through cosmetics contribute to public health.
- Promoting Fair Competition: The FDA’s regulations help create a level playing field for manufacturers, preventing unfair competition based on misleading claims or unsafe practices.
FAQs: Common Questions about FDA Requirements for Skin Care Products
Q1: Does the FDA approve skin care products?
A: The FDA does not generally approve cosmetics, including skin care products. However, the agency retains the authority to take action against products that pose a health risk or are mislabeled.
Q2: What are the consequences of non-compliance with FDA regulations?
A: Non-compliance with FDA regulations can lead to various consequences, including:
- Warning letters: The FDA may issue warning letters to manufacturers who are in violation of regulations.
- Product seizures: The FDA can seize products that are deemed unsafe or mislabeled.
- Civil and criminal penalties: Manufacturers who violate FDA regulations may face civil or criminal penalties, including fines and imprisonment.
Q3: How can I report a problem with a skin care product?
A: If you suspect a skin care product is unsafe or mislabeled, you can report it to the FDA through its website or by phone.
Q4: How can I ensure that the skin care products I use are safe?
A: You can take several steps to ensure that the skin care products you use are safe:
- Read product labels carefully: Pay attention to the ingredients list, intended use, and any warnings.
- Do your research: Look for products that have been tested and are backed by scientific evidence.
- Be cautious of claims: Be skeptical of products that make exaggerated or unsubstantiated claims.
- Check for FDA warnings: The FDA publishes warnings about products that have been found to be unsafe.
- Talk to your doctor: If you have any concerns about a skin care product, talk to your doctor.
Tips for Manufacturers: Ensuring Compliance with FDA Requirements
- Know the regulations: Thoroughly understand the FDA’s regulations for cosmetics and skin care products.
- Develop a compliance program: Implement a comprehensive program to ensure that your products meet FDA requirements.
- Test your products: Conduct appropriate testing to ensure the safety and efficacy of your products.
- Label your products accurately: Ensure that your product labels are truthful and comply with FDA requirements.
- Keep good records: Maintain detailed records of your manufacturing processes, testing results, and product labeling.
- Stay informed about changes in regulations: The FDA may update its regulations periodically. Stay informed about these changes to ensure ongoing compliance.
Conclusion: A Shared Responsibility for Safe and Effective Skin Care
The FDA’s regulatory framework for skin care products is a vital safeguard for consumers, ensuring that products are safe and marketed truthfully. While manufacturers bear the primary responsibility for compliance, consumers also play a crucial role in protecting their health by being informed and making responsible choices. By working together, manufacturers, consumers, and the FDA can contribute to a safer and more effective skin care industry.






Closure
Thus, we hope this article has provided valuable insights into Navigating the Regulatory Landscape: FDA Requirements for Skin Care Products. We appreciate your attention to our article. See you in our next article!